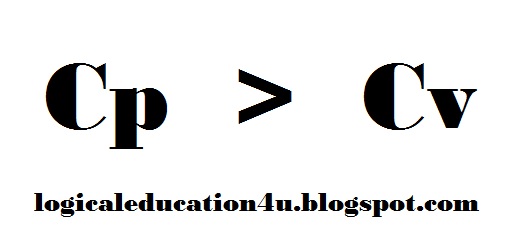
Relation Between Cp & Cv
to understand the relation between Cp and Cv we must know that what they are:
Cp:
The amount of heat required to raise the temperature of a system at constant pressure.Cv:
The amount of heat required to raise the temperature of a system at constant volume.
RELATION:
If the system is set at constant pressure and heat is supplied to it according to first law of thermodynamics that heat converted into some amount of work and its internal energy increases and then temperature
Amount of heat(Q) = Use ful Work(W) + Raise in internal energy(U)
If the system is set at constant volume and then heat is supplied
to it according to first law of thermodynamics no work be there and all heat
energy is used to increase its internal energy and temperature.
Amount of heat(Q) = Raise in internal energy(U)
the raise in internal energy is the raise in temperaure.
Because internal energy is directly proportional to the absolute temperature.











0 comments