DOWN'S PROCESS
INTRODUCTION:
J.C Down developed a process for the manufacture of sodium metal known as DOWN's Process.
PRINCIPLE:
The process based upon the principle of electrolysis of Aqeous sodium chloride solution
NaCl → 2Na + Cl2
RAW MATERIALS:
- Fused sodium chloride is used as the electrolyte in the process.
- As the sodium chloride is has the melting point of 801 degree celcius some amount of calcium chloride is added to lower the melting point to about 600 degree celcius which makes the process feasible.
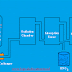
Working:
When an electric current is passed through the molten mixture of NaCl and CaCl2, NaCl decomposes in to Na+ and Cl- ion. Na+ ions migrate towards cathode while Cl- ions towards the anode. The molten sodium collects in the cathode compartment where it rises to the top and is tapped off by a pipe. Chlorine is collected at the anode.
ELECTROCHEMICAL CHANGES
At cathode
Na+-ions migrate to cathode where they are reduced to Na.
2Na+ + 2e → 2Na (Reduction)
At anode
Cl--ions migrate to anode and oxidised to form chlorine gas.
2Cl- → Cl2 + 2e- (Oxidation)
Overall Reaction
2Na+ + 2e- → 2Na
2Cl- → Cl2 + 2e-
__________________
2Na+ + 2Cl- → 2Na + Cl2
During electrolysis calcium is also obtained at cathode but sodium and calcium are separated from each other due difference in density. Density of Na is 0.67gm/cc and the density of Ca is much higher than that of Na i.e. 2.54gm/cc. That's why they do not mix with each other.
For latest information , free computer courses and high impact notes visit www.logicaleducation4u.ml