Oxides of group 2 were originally thought to be elements, not compounds. These "elements" were called alkaline earths: "alkaline" because they're basic when added to water, and 'earth' because that's what they called oxides that they thought were elements.
Wednesday, 24 August 2016
The Group IA elements are called alkali because when they reacts with water they forms basic solutions like Na metal reacts with water and form NaOH a strong base.
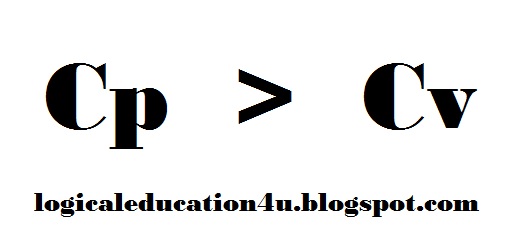
Relation Between Cp & Cv
to understand the relation between Cp and Cv we must know that what they are:
Cp:
The amount of heat required to raise the temperature of a system at constant pressure.Cv:
The amount of heat required to raise the temperature of a system at constant volume.
RELATION:
If the system is set at constant pressure and heat is supplied to it according to first law of thermodynamics that heat converted into some amount of work and its internal energy increases and then temperature
Amount of heat(Q) = Use ful Work(W) + Raise in internal energy(U)
If the system is set at constant volume and then heat is supplied
to it according to first law of thermodynamics no work be there and all heat
energy is used to increase its internal energy and temperature.
Amount of heat(Q) = Raise in internal energy(U)
the raise in internal energy is the raise in temperaure.
Because internal energy is directly proportional to the absolute temperature.
Sunday, 21 August 2016
35 isomers of nonane:
- Nonane
- 2-methyloctane
- 3-methyloctane
- 4-methyloctane
- 2,2-dimethylheptane
- 2,3-dimethylheptane
- 2,4-dimethylheptane
- 2,5-dimethylheptane
- 2,6-dimethylheptane
- 3,3-dimethylheptane
- 3,4-dimethylheptane
- 3,5-dimethylheptane
- 4,4-dimethylheptane
- 3-ethylheptane
- 4-ethylheptane
- 2,2,3-trimethylhexane
- 2,2,4-trimethylhexane
- 2,2,5-trimethylhexane
- 2,3,3-trimethylhexane
- 2,3,4-trimethylhexane
- 2,3,5-trimethylhexane
- 2,4,4-trimethylhexane
- 3,3,4-trimethylhexane
- 2-methyl-3-ethylhexane
- 2-methyl-4-ethylhexane
- 3-methyl-3-ethylhexane
- 3-methyl-4-ethylhexane
- 2,2,3,3-tetramethylpentane
- 2,2,3,4-tetramethylpentane
- 2,2,4,4-tetramethylpentane
- 2,3,3,4-tetramethylpentane
- 2,2-dimethyl-3-ethylpentane
- 2,3-dimethyl-3-ethylpentane
- 2,4-dimethyl-3-ethylpentane
- 3,3-diethylpentane
Thursday, 18 August 2016
INTRODUCTION:
It is the fact that the gases have not fixed volume or shape and their volume can be change by the increase or decrease the pressure or temperature.the behaviour of a gas is described by the four variables pressure, volume, temperature and the mass of a gas.
STATEMENT:
"For a given mass of a gas the volume is inversely proportional to the pressure exerted by it when the temerature of a gas is kept constant"
MATHEMATICALLY :
Let 'V' is the Volume of a gas which exerts a pressure 'P' on its surrounding at constant.
Then According to the Boyle's Law
It is the fact that the gases have not fixed volume or shape and their volume can be change by the increase or decrease the pressure or temperature.the behaviour of a gas is described by the four variables pressure, volume, temperature and the mass of a gas.
BOYLES's LAW:
INTRODUCTION:
The law was given by Robert Boyle in 1660. He found experimently the relation between the Pressure and Volume at constant Temperature.
"For a given mass of a gas the volume is inversely proportional to the pressure exerted by it when the temerature of a gas is kept constant"
MATHEMATICALLY :
Let 'V' is the Volume of a gas which exerts a pressure 'P' on its surrounding at constant.
Then According to the Boyle's Law
V α 1/P
OR
VP= Constant
that is the product of the pressure and volume is constant at constant temerature.
V3=Volume at 3rd state
VN=Volume at Nth state
if the mass of gas is not kept constant.
FOR N states of a GAS:
Consider N states of gas where the pressure and volume changes but temperature is kept constant.
P1V1=P2V2=P3V3..............PNVN
Where:
P1=Pressure at 1st state
P2=Pressure at 2nd state
P3=Pressure at 3rd state
PN=Pressure at Nth state
V1=Volume at 1st state
V2=Volume at 2nd state
V3=Volume at 3rd state
VN=Volume at Nth state
if the mass of gas is not kept constant.
P1V1/m= P2V2/m = P3V3/m =............. PnVn/m
where 'm' is the mass of a gas.
GRAPHICALLY:
let we take pressure at Y-axis and the Volume at X-axis then the Graph will be:
The Hyperbolic Curve is formed.
CHARLES LAW:
INTRODUCTION:
The law is given by CHARLES as known as the charles law which gives the relation between the volume and Temperature at Constant Pressure.
STATEMENT:
"For a given mass of a gas the volume is directly proportional to the temperature of a body when the pressure is kept constant"
MTHEMATICALLY:
let us consider a gas has a volume 'V" and at temerature 'T' where as the pressure of the gas is kept constant"
then according to the charles law:
V α T
OR
Wednesday, 17 August 2016
DEFINATION:
"The expansion in solids is due to the absorption of heat is known as thermal expansion in solids"
Types:
- linear thermal Expansion
- Surface thermal Expansion
- Volumetric thermal Expansion
LINEAR THERMAL EXPANSION:
"the expansions in either lenght or breadth or height of an isotropic solid due to the absorption of heat is known as the linear
thermal expansion in solids"SURFACE THERMAL EXPANSION:
"the expansion in the surface area of a solid due to the absorption of heat is known as surface thermal expansion in solids"
VOLUMETRIC THERMAL EXPANSION:
"The expansion in the volume of a solid due to absorption of heat is known as the volumetric thermal expansion in solids"
TEMPERATURE:
Defintion:
"The Degree of Hottness or Coldness of a body is called it's Temperature"
E.g:
- 500 Celcius(degree of hottness)
- -10 Celcius(degree of coldness)
SCALES OF MEASURING TEMPERATURE:
The Relation Between these scales are
(Temperature in C) - 0 (Temperature in F) - 32 (Temperature in K) - 273
------------------------- = --------------------------- = ----------------------------
100 180 100
------------------------- = --------------------------- = ----------------------------
100 180 100
Tuesday, 16 August 2016
OLD CONCEPT OF CLASSICAL PHYSICS :
Upto the begening Century the heat is called "A weightless fluid called coloric which is present in every material body and only emits out of a substance when it is being cut or burnt"
After some years the fluid concept is challanged by Count Rumford.
He made an experiment in order to broke that concept of classical physics he take a cannon barrels and a very dull drill and tried to cut that but it not cut but it is very heated so the concept that the (heat is only emit out of a substance when it is being cut or burnt) was discarded.There become a little Difference in the defination of heat that:
"the heat is emit out of a substance when the machenical work or pressure is in progress"
"the heat is emit out of a substance when the machenical work or pressure is in progress"
MODERN CONCEPT :
In 19th Century the heat is defined as:
"The HEAT is a form of ENERGY that transfers from the hot body to a cold body"
When a hot body get in thermal contact with the cold body the heat transfers from the hot body to the cold body and the cold body absorbs the heat and then the heat is converted to its Internal Energy.
Internal Energy:
"It is the Sum of microscopic kinetic and Potential Energies of the molecules of the system"